The cotton warp I described in Slow Cloth, well-travelled is woven, off the loom, finished. 6.18m/6.75yds of fabric c. 9″/23mm wide. Cotton singles warp, silk and cotton singles weft, all dyed in a (fructose, from memory) indigo vat. Note that the warp stripes are unplanned and result from differential dyeing by the different colours and types of cotton.
A couple of people have asked for more technical information about the yarn (wpi, tpi). Remember that this was random spinning over the last 5 years or more, not directed spinning for the project. Cottons include pima, supima, organic green and brown, spun from punis, top and directly from the seeds, on tahklis, charkha, Majacraft quill and lace flyer. All were spun clockwise, or z. The yarn(s) vary widely in grist. In the photo below of remnants of the sized warp the 1/2″ wide sample is wrapping at roughly 52-56wpi. In the 1/8″ sample the yarn is wrapping at over 70wpi. (The greenish colour is indigo over natural brown, which together with its thinness tells me this was probably tahkli spun from seeds on my lap in front of the tv. Why weave your handspun? For the memories.)

Twist per inch is also variable. It is difficult to see the twist in the sized yarn, but examining unsized yarn in the weft bag – the warp was selected randomly from the skeins of dyed yarn, so both are equally variable – with a magnifier I eyeballed twist from roughly 45° to 55–60°, assuming 90° is perpendicular to the length of yarn. So it’s reasonably high twist… and that’s AFTER boiling. The yarn was probably fractionally thicker before boiling, so add something to those twist angles for twist on the singles as they came off the bobbin/shaft. Basically, sample. That’s what this fabric is, a jacket-length sample.
I like it.

That’s the fabric finished to my current satisfaction. An overnight soak followed by wool/handwash cycle did not remove the gelatine size, so I soaked this in a bucket of the hottest water from the tap plus a couple of kettles of boiling water, washed on permanent press ‘warm’, then ironed on hottest cotton setting. It’s shrunk about 10% in width. I like it a lot. Despite the cursing and frustration, I also appreciate the lessons it has taught me. Some are unlikely to be needed again, such as: Do not expect a warp on the loom to make an international move without shifting somewhat. Others may be useful time and again.
My handspun cotton weaves well, but beware of slubs.
Some of my earliest and most unevenly spun cotton singles are in this warp. Only the very thinnest singles snapped under the quite high weaving tension, and by ‘very thinnest’ I mean places where uneven singles were thinner than fine sewing thread so I confess I’m feeling smug and planning a super-thin cotton fabric. But thick slubs are as bad or even worse than thin spots: the slubs hold more size, so are stiffer and fail to bend around the weft. They stuck up out of the fabric a bit after weaving and some are still visible after finishing. I can live with this.

Most of my stupid mistakes occurred during the weaving; I’m even more proud of my spinning because the singles stood up to such mistreatment.
Wind sized yarns from the skeins onto bobbins before winding the warp.
See the underline? That means this one’s particularly important. Because winding the warp from a skein held on a flexing umbrella swift with occasional pauses to break the yarn free from places where the size has stuck threads together is not exactly conducive to even warp tension, is it?
I am rubbish at winding warps.
Perhaps fractionally less rubbish now because I’ve wound a few since this one, but still. This warp was wound one thread at a time from two different umbrella swifts over the course of three days. Should I be surprised that I had to hang a chiming array of weights from slack threads? No, I should not.
Thoroughly set the weft twist before weaving.
Or be prepared to guard against pigtails forming when you slacken tension to beat it into place. I am told that many traditionally-spun and woven cotton fabrics from South America have these pigtails, so it’s authentic, but still. I prefer my fabric without.
Be open-minded about lease sticks in or out while weaving.
The lease sticks were useful for this warp, seeming to help even out the tension. But on earlier fuzzy warps (cashmere and fine merino handspun lace) the lease sticks seemed to raise the fuzz, encouraging the the ends to stick together.
But enough criticism.

The fabric is slightly stiff even after the size has been washed out, reminiscent of the stiffness of new denim in the days before lycra. Not as stiff as those old new jeans – it’s a much thinner fabric – but similar. I’m guessing I’ve achieved my goal of spinning and weaving a cloth that will soften with wear like the denim of yore.
The warp was threaded for point twill; the fabric shows the three different weaves. To right plainweave/tabby; centre first twill (Strickler p.28 #94, Point twill from A German Weaver’s Pattern Book 1784–1810); left 8-shaft diamond twill. (The red line marks the point where I changed the tie-up from first to second twill.) The width of the fabric decreased between patterns as the interlacement changed; the first twill (#94) is narrower than plainweave as the twill pattern packs threads more densely. The second twill, 8-shaft diamond, has no band of plainweave so packs even more tightly for a fabric slightly narrower than the first twill. The silk weft and denser packing of the twills makes both heavier fabrics, but not dangerously dissimilar to the plainweave with cotton weft.
The plainweave shows the warp striping most clearly and, like the wool singles fabrics I have woven, it is a little more elastic than a fabric woven from plied yarn. It’s heavy shirting in weight, noticeably lighter in the hand than either twill. To be expected, but it’s interesting to have it confirmed.
The patterning of twill #94 is less obvious in the fabric than I’d hoped. It’s there if you look closely, though.
Once again 8-shaft diamond twill is just right, at least as far as I’m concerned. The fabric has the hand of lightweight denim. It looks good with the silk weft.

But I think it looks even better with the slightly paler blue cotton I used when I finished the silk (or at least couldn’t find any more).

The pale blue fades in and out of the warp cottons, the diamonds seeming to shimmer in the fabric. It’s country cloth, but it’s interesting. I should play with the effect, I like it so much. I wish I had more than 24cm.
So. What am I going to do with it? Being a process rather than project person I’d really love to put it away and make the next fabric, but that defeats the purpose somewhat. There is more to be learned from this sample: I need to know how it wears. I could just bundle it into a bag and handle it until it softens but really, it’s fabric. It should be a garment. A sample can be a jacket or a shirt, and I think that’s what this will be once I overcome my fear of making garments. Why spin and weave? To make a garment from cloth you cannot buy, where one warp makes three different patterns for different parts of the garment. Eventually!



 Indigo-dyed handspun cotton singles drying on the rosemary bush by the kitchen door. I miss that bush, and our North Ronaldsay sky-blue doors.
Indigo-dyed handspun cotton singles drying on the rosemary bush by the kitchen door. I miss that bush, and our North Ronaldsay sky-blue doors. I had to finish drying the yarns on the radiator. Hmm. Clearly I dyed silk fibre, too. I wonder where that is? In one of the many boxes, of course.
I had to finish drying the yarns on the radiator. Hmm. Clearly I dyed silk fibre, too. I wonder where that is? In one of the many boxes, of course. The gelatin-sized yarns drying under light tension – those water bottles are not full! – suspended between the hoe and an old rake handle wrapped in clingfilm aka Saran wrap on the clothes tree. The old grey lunchbag contains clothes pegs.
The gelatin-sized yarns drying under light tension – those water bottles are not full! – suspended between the hoe and an old rake handle wrapped in clingfilm aka Saran wrap on the clothes tree. The old grey lunchbag contains clothes pegs.

 I have started weaving some fine yarns with the lease sticks in place. Like back-to-front or front-to-back warping, lease sticks in or out is something weavers seem to choose as habit early in their careers. I didn’t like the lease sticks in when weaving thicker, fuzzy yarns: my impression was that the sticks were encouraging fuzziness and binding of threads in the warp. But feeding firmly sized, finer yarns through the even tension of the lease sticks seems to correct some of my warping issues.
I have started weaving some fine yarns with the lease sticks in place. Like back-to-front or front-to-back warping, lease sticks in or out is something weavers seem to choose as habit early in their careers. I didn’t like the lease sticks in when weaving thicker, fuzzy yarns: my impression was that the sticks were encouraging fuzziness and binding of threads in the warp. But feeding firmly sized, finer yarns through the even tension of the lease sticks seems to correct some of my warping issues. 





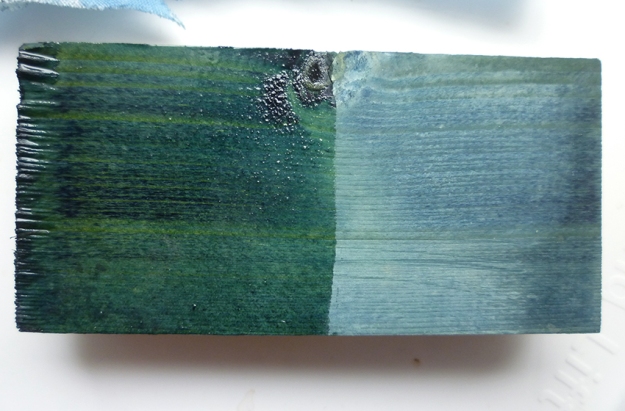
 And here is the result, reskeined with the Ditchling Museum Project Record. I’ve added a bobbin of cotton dyed with with an ‘ordinary’ indigo vat for comparison: it’s clear to my eyes at least that the Saxon Blue is more teal, somewhat greener than the ordinary. But sadly not nearly as green as the example I saw produced by a friend with access to Fuming Sulphuric: the stronger the acid, the higher the proportion of green ‘sulph-indylic’ dye formed by the reaction. But still, it is very pretty.
And here is the result, reskeined with the Ditchling Museum Project Record. I’ve added a bobbin of cotton dyed with with an ‘ordinary’ indigo vat for comparison: it’s clear to my eyes at least that the Saxon Blue is more teal, somewhat greener than the ordinary. But sadly not nearly as green as the example I saw produced by a friend with access to Fuming Sulphuric: the stronger the acid, the higher the proportion of green ‘sulph-indylic’ dye formed by the reaction. But still, it is very pretty.